29 September to 3 October 2024
Coordinators: Eric J. Kremer (CNRS, IGMM Université de Montpellier, FR), Joaquim Vives (Banc de Sang I Teixits, ES), Margarida Serra (iBET, PT)
Applications are now closed. All applicants will be informed of their acceptance to the courses by end of July at the latest.
Coursebook LINK
Gene and cell products are paving the way towards more effective therapeutics for unmet medical needs. Currently, one of the main translational challenges is a consistent and cost-effective supply of these complex medicinal products. Taking into account the interest raised by ESACT members and other learned societies, we are organizing the 4th Bioprocessing and Manufacturing of Gene and Cell Therapy Products Course in Llafranc, Costa Brava/Spain, in September 2024. ESACT is introducing this activity as one more contribution to the community targeting the use of viral vectors, stem cells and immune cells for therapy. This course is intended for Ph.D. students, post-docs, junior scientists, engineers and clinicians searching to improve their understanding of the development and manufacturing of gene and cell therapy products.
GRANTS
A limited number of grants, covering the course fee are provided by ESACT and ACTIP. Applicants to the grants should indicate it in the course application, together with a motivation statement. Priority will be given to young PhD students from Academia but ACTIP will provide several grants for participants from industry, too.
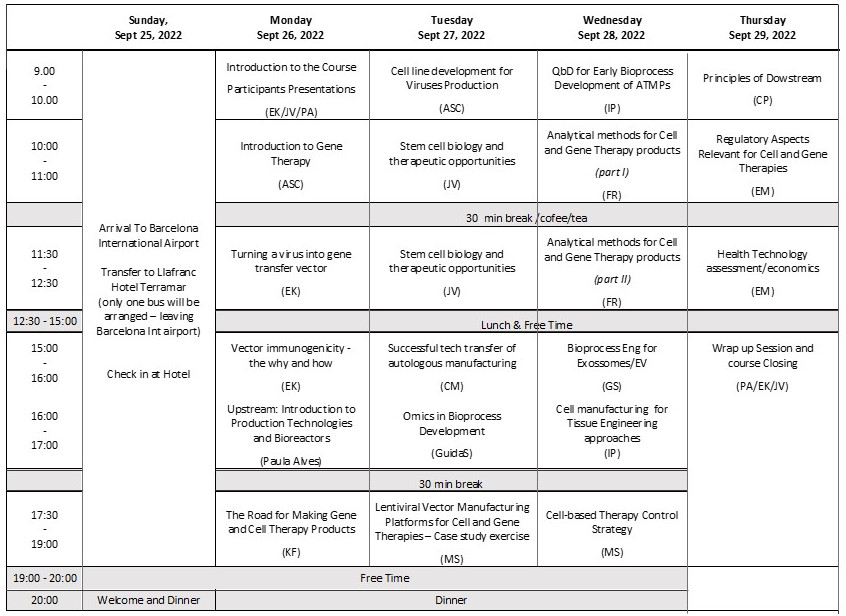
LECTURES
The speakers will address key bioprocessing aspects, analytical toolsets to assess the quantity and quality attributes, and regulatory challenges for the manufacturing of ATMPs, providing also a solid fundamental basis on vectorology and stem cell biology. The course is designed as an interactive 5-day program with the number of participants restricted to 30 to foster interaction among speakers and attendees. The course comprises lectures covering the main topics of Gene & Cell Therapy:
The course comprises lectures covering the main topics of Animal Cell Technology:
- Introduction to Gene Therapy & to Cell Therapy
- Immunology, Virology & Vectorology
- Cell Line Development for Viruses Production
- Stem Cell Biology
- Gene Modification of Cells for Therapy
- Bioreactor scale-up, scale down and single use Bioreactor for Cells based Products & Viral Vectors
- Downstream processing for Cells based Products & Viral Vectors
- Omics in Bioprocess Development
- Manufacturing Cells based Products & Viral Vectors
- Novel Modalities in Gene & Cell Therapy
- Scale-up and the Role of Automation in Gene & Cell Therapy
- Process & Product Analytics
- Health Technology Assessment/Economics
- Decision Tools
The program has also slots dedicated to presentation of case studies by lecturers, workshops, exercises and discussion groups with the lecturers.
Lecturers: Eric Kremer (EK; CNRS, France), Joaquim Vives (JV; BST, Spain), Margarida Serra (GS; iBET, Portugal), Ana Coroadinha (AC; ITQB NOVA, Portugal), Chantal Martin (CM; Turnstone, Canada), Eoin McGrath (EM; ICCBBA, USA/Spain), Ioannis Papantouniou (IP; KU Leuven, Belgium), Kerry Fisher (KF; Univ. Oxford, UK), Mercedes Segura (MS; ElevateBio, USA), Dominik Bruecher (Vector Biopharma, CH).
Organizing Committee
Paula Marques Alves
Animal Cell Technology Unit
IBET/ITQB-UNL
Apartado 12, 2780-901 Oeiras
Portugal
Tel.: +351 21 4469421
E-mail: marques@ibet.pt
Joaquim Vives
Servei de Teràpia Cellular
Banc de Sang i Teixits
Edifici Doctor Frederic Duran i Jordà
Passeig Taulat, 116
08005 Barcelona
Tel.: +34 93 557 35 00 (ext 6708)
E-mail: jvives@bst.cat
Eric J Kremer
CNRS, IGMM – Institut de Génétique Moléculaire de Montpellier
France
1919 rt de Mende
Montpellier, 34293
France
Tel.: +33 4 34 35 96 72 (lab 74)
E-mail: eric.kremer@igmm.cnrs.fr
Contact for registration
Birgit Marckhgott
E-mail: office@esact.org
Organizing Committee
Margarida Serra
Animal Cell Technology Unit
iBET/ITQB-UNL
Apartado 12, 2780-901 Oeiras
Portugal
Tel.: +351 21 4469431
E-mail: mserra@ibet.pt
Joaquim Vives
Servei de Teràpia Cellular
Banc de Sang i Teixits
Edifici Doctor Frederic Duran i Jordà
Passeig Taulat, 116
08005 Barcelona
Tel.: +34 93 557 35 00 (ext 6708)
E-mail: jvives@bst.cat
Eric J Kremer
CNRS, IGMM – Institut de Génétique Moléculaire de Montpellier
France
1919 rt de Mende
Montpellier, 34293
France
Tel.: +33 4 34 35 96 72 (lab 74)
E-mail: eric.kremer@igmm.cnrs.fr
Contact for registration
Ms. Marie Groll & Ms. Marierose von Ledebur
Remember Management GmbH
E-mail: office@esact.org
Organizing Committee
Margarida Serra
Animal Cell Technology Unit
iBET/ITQB Universidade Nova de Lisboa
Apartado 12
2780-901 Oeiras Portugal
Tel.: +351 21 4469431
E-mail: mserra@ibet.pt
Joaquim Vives
Servei de Teràpia Cellular
Banc de Sang i Teixits
Edifici Doctor Frederic Duran i Jordà
Passeig Taulat 116
08005 Barcelona, Spain
Tel.: +34 93 557 35 00 (ext 6708)
E-mail: jvives@bst.cat
Eric J. Kremer
CNRS, Institut de Génétique Moléculaire de Montpellier (IGMM)
Université de Montpellier
1919 rt de Mende
34293 Montpellier, France
Tel.: +33 4 34 35 96 72 (lab 74)
E-mail: eric.kremer@igmm.cnrs.fr
Contact for registration
Ms. Marie Groll & Ms. Marierose von Ledebur
Remember Management GmbH
E-mail: office@esact.org
Application Form
Application for 2023 is open until 15 June – apply now!
By filling out and submitting the contact form below, you are applying to join the course. The selection of participants will be based solely on the strength of your motivation letter and your CV (in case of applying for a grant). It does not depend on the date of receipt of the application.